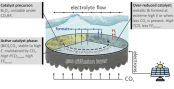
CO2 Conversion at High Current Densities: Stabilization of Bi(III)-Containing Electrocatalysts under CO2 Gas Flow Conditions
The paper authored by I. Zelocualtecatl Montiel, A. Dutta, K. Kiran, A. Rieder, A. Iarchuk,
S. Vesztergom,
M. Mirolo, I. Martens, J. Drnec and P. Broekmann
is published in ACS Catalysis (2022, vol. 12, pp. 10872-10886).
Abstract:
Herein, we demonstrate the superior performance of bismuth subcarbonate ((BiO)2CO3) layer catalysts for formate production using a fluidic CO2-fed electrolyzer device. The subcarbonate catalyst readily forms in situ from a CO2-absorbing Bi2O3 precursor material during the CO2 reduction reaction (CO2RR). In 1 mol dm–3 KOH electrolyte solution, a maximum Faradaic efficiency of FEformate = 97.4% (corresponding partial current density of formate formation: PCDformate = −111.6 mA cm–2) was achieved at a comparably low applied electrolysis potential of −0.8 V vs the reversible hydrogen electrode (RHE). Even higher values of PCDformate = −441.2 mA cm–2 (FEformate = 62%) were observed at a more cathodic potential, −2.5 V vs RHE. As the alkalinity of the liquid electrolyte is further increased (e.g., by using 5 mol dm–3 KOH solution), the performance of formate production is boosted beyond PCDformate values of −1 A cm–2. Combined X-ray diffraction and Raman spectroscopic investigations demonstrate an extraordinarily high stability of Bi(III) cations in the catalytically active subcarbonate catalyst phase down to cathode potentials of −1.5 V vs RHE. This stabilization effect can clearly be attributed to the high abundance of gaseous CO2 under the operating conditions of the gas-fed electrolyzer. In the absence of any CO2 supply, the reductive Bi(III) → Bi(0) transition already occurs under much milder conditions of −0.3 V vs RHE, as evidenced by in situ Raman spectroscopy in CO2-free 1 mol dm–3 KOH electrolyte solution. An advanced X-ray diffraction computed tomography technique was applied to gain deeper insights into the spatial distribution of the metallic and subcarbonate phases comprising the active composite catalyst layer during the CO2RR.
